It doesn’t matter when you read this: EudraVigilance or VAERS data never represent the vaccine’s actual side effects
Graphics indicating that covid-19 vaccines supposedly cause numerous adverse side effects have resurfaced on social media platforms

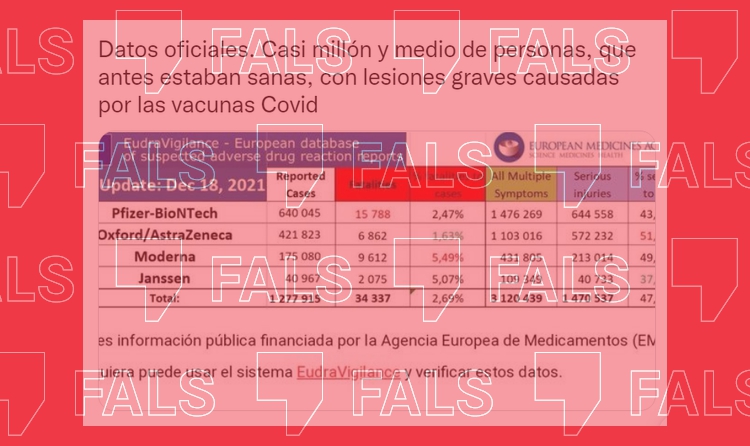
Graphics indicating that covid-19 vaccines supposedly cause severe and numerous adverse side effects in people who had previously been in good health have resurfaced on social media platforms. In this instance, the information was shared by Ángeles Maestro, a Spanish politician and one of the founders of the Izquierda Unida party (English: United Left), whose tweet received over 800 retweets and 1,000 likes.
However, similar to many other cases where EudraVigilance data is used, the conclusion drawn from them is wrong: there are not almost 1.5 million people who have suffered serious injuries caused by covid-19 vaccines. EudraVigilance is a European pharmacovigilance system that informs the public of adverse events that coincide with the time that a medication was administered (in this case, the covid-19 vaccine). But it does not imply causation in any case. That holds true for all graphics on social media platforms where data taken from this database – or from VAERS, the equivalent system in the US with a similar logic – are shared.
Official data. Almost 1.5 million people, previously in good health, have suffered serious injuries caused by covid-19 vaccines.
It doesn’t matter when you read this: neither the US database VAERS (Spanish only) nor the European database EudraVigilance (Spanish only) shows what adverse side effects the covid-19 vaccine produces, how many of them there are or how frequently they occur. What they do show —which is reflected on each of their websites (here and here)— are suspected adverse events that occur after any sort of drug (including covid-19 vaccines) has been administered, and which may or may not be related to the administration of said drug.
So saying that “almost 1.5 million people who had previously been in good health” now have “serious injuries caused by covid vaccines” is incorrect: just because that number of people reported an adverse event after getting the jab does not mean those events are all associated with the vaccine.
What purpose do these databases serve?
In reality, the data presented on EudraVigilance and VAERS do not say anything about the vaccines themselves. Still, it is important to analyse them since they make it possible for regulatory agencies to detect whether a specific event occurs more frequently than normal.
Thanks to the notifications sent to these databases, experts were able to identify thrombosis (Spanish only) and myocarditis as adverse side effects of the adenovirus and mRNA vaccines, respectively, and they are now listed as potential adverse side effects in the vial package inserts.
In conclusion, these databases are another tool to monitor the vaccines, but the European site warns that it has limited control over the accuracy of the data it receives.
If the data cause confusion, why are they made public?
It is not the first time that Verificat and other fact-checking agencies have verified messages based on data from EudraVigilance or VAERS. The information these databases provide is open and freely accessible to the public, which can lead to misinterpretations, as is the case with some of the content circulating on the internet.
It is therefore important to know how to interpret the purpose of these data and to refrain from spreading messages that contain inaccurate conclusions based on them. And to always remember that correlation does not imply causation.
How can I report an adverse event?
It depends on where you live. Reporting works differently in the US and Europe: in the American system, US citizens can submit data themselves via this website, while in the European system, data normally come from pharmaceutical laboratories and the individual drug regulation authorities of each country.
In some places, like Spain (Spanish only) or France (French only), citizens can report adverse events directly to the country’s authorities without prior consultation with health experts. These authorities in turn submit the data to the European platform.
Unlike the VAERS portal in the US, it is not possible to report cases directly to EudraVigilance.